- Scenario five – analysing the ABG results

Feedback
That is not quite right. Have another go.
Feedback
That is not quite right.
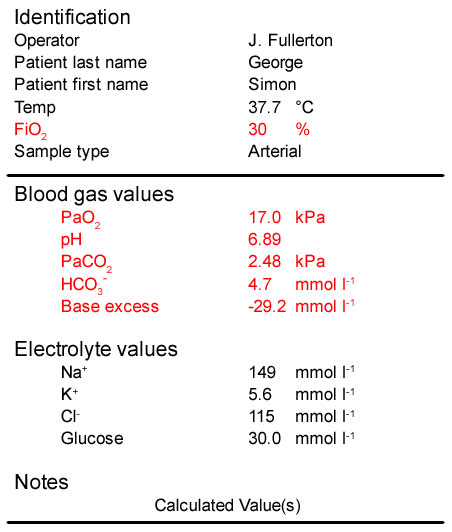
- The patient’s PaO2 is 17 kPa. This is >10 kPa and is consistent with breathing 30% oxygen. His oxygenation is not impaired
- His pH is well below the lower limit of normal (7.35) and he has a very severe acidaemia
- His PaCO[sub]2[/sub] is low and is not consistent with his pH. This is due to his respiratory system trying to compensate for his acidaemia by hyperventilating and removing CO[sub]2[/sub]. He therefore has a respiratory alkalosis
- He has a massive reduction in bicarbonate (and associated fall in base excess) which has been consumed to buffer the production of ketoacids. He therefore has a severe metabolic acidosis
The patient’s blood glucose is also elevated at 30 mmol l-1 and his urine is strongly positive for ketones.
In summary:
These blood gas results are consistent with severe diabetic ketoacidosis. Further evidence is the presence of ketones in his urine and the very high blood glucose. There is a primary metabolic acidosis with partial compensation provided by the respiratory alkalosis.
Treatment will include:
- Fluid resuscitation, initially with normal saline
- Insulin, with regular measurement of blood glucose
The use of bicarbonate is controversial but many clinicians would give it in the presence of such a severe acidaemia, particularly if it did not improve rapidly after starting the above measures.
Feedback
That is not right. Have another go.
Feedback
That is not right.
- The patient’s PaO2 is 17 kPa. This is >10 kPa and is consistent with breathing 30% oxygen. His oxygenation is not impaired
- His pH is well below the lower limit of normal (7.35) and he has a very severe acidaemia
- His PaCO[sub]2[/sub] is low and is not consistent with his pH. This is due to his respiratory system trying to compensate for his acidaemia by hyperventilating and removing CO[sub]2[/sub]. He therefore has a respiratory alkalosis
- He has a massive reduction in bicarbonate (and associated fall in base excess) which has been consumed to buffer the production of ketoacids. He therefore has a severe metabolic acidosis
The patient’s blood glucose is also elevated at 30 mmol l-1 and his urine is strongly positive for ketones
In summary:
These blood gas results are consistent with severe diabetic ketoacidosis. Further evidence is the presence of ketones in his urine and the very high blood glucose. There is a primary metabolic acidosis with partial compensation provided by the respiratory alkalosis.
Treatment will include:
- Fluid resuscitation, initially with normal saline
- Insulin, with regular measurement of blood glucose
The use of bicarbonate is controversial but many clinicians would give it in the presence of such a severe acidaemia, particularly if it did not improve rapidly after starting the above measures.
Feedback
That is right.
- The patient’s PaO2 is 17 kPa. This is >10 kPa and is consistent with breathing 30% oxygen. His oxygenation is not impaired
- His pH is well below the lower limit of normal (7.35) and he has a very severe acidaemia
- His PaCO[sub]2[/sub] is low and is not consistent with his pH. This is due to his respiratory system trying to compensate for his acidaemia by hyperventilating and removing CO[sub]2[/sub]. He therefore has a respiratory alkalosis
- He has a massive reduction in bicarbonate (and associated fall in base excess) which has been consumed to buffer the production of ketoacids. He therefore has a severe metabolic acidosis
The patient’s blood glucose is also elevated at 30 mmol l-1 and his urine is strongly positive for ketones.
In summary:
These blood gas results are consistent with severe diabetic ketoacidosis. Further evidence is the presence of ketones in his urine and the very high blood glucose. There is a primary metabolic acidosis with partial compensation provided by the respiratory alkalosis.
Treatment will include:
- Fluid resuscitation, initially with normal saline
- Insulin, with regular measurement of blood glucose
The use of bicarbonate is controversial but many clinicians would give it in the presence of such a severe acidaemia, particularly if it did not improve rapidly after starting the above measures.
References
Essentials: 5-step approach to ABG interpretation
Step 2 – is the patient hypoxaemic?
Step 3 – is the patient acidaemic or alkalaemic?
Step 4 – what happened to the PaCO2?
Step 5 – what has happened to the base excess or bicarbonate?
Normal values
PaO[sub]2[/sub] > 10 kPa (75 mmHg) on air
pH 7.35 – 7.45
PaCO[sub]2[/sub] 4.7 – 6.0 kPa
HCO[sub]3[/sub] 22 – 26 mmol l-1
BE +/- 2 mmol l[sup]-1[/sup]