- Scenario three – analysing the ABG results

Feedback
That is not quite right. Have another go.
Feedback
That is not quite right.
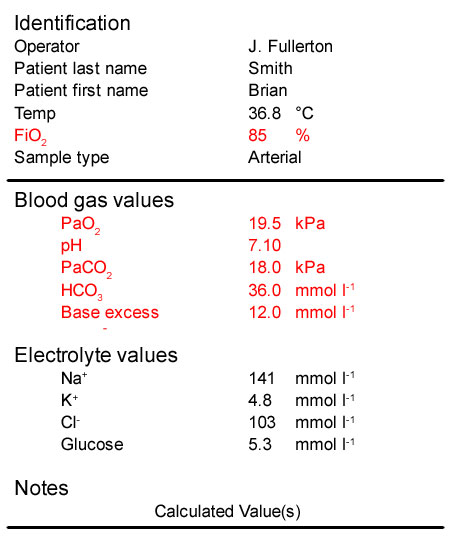
- The patient’s PaO2 is >10 kPa indicating he is not hypoxaemic. However, we would expect a much higher PaO[sub]2[/sub] being ventilated with 85% oxygen. This indicates severely impaired oxygenation
- His pH is 7.10, much lower than normal indicating that he has an acidaemia
- His PaCO[sub]2[/sub] is very high which is consistent with his low pH and so he has a respiratory acidosis. This is due to the period of apnoea and his underlying COPD
- His bicarbonate and base excess are both increased, indicating a metabolic alkalosis. This is a compensatory mechanism for his longstanding COPD. Prior to his respiratory arrest this compensation would have resulted in his pH being almost normal despite an increased PaCO[sub]2[/sub]
In summary:
The significant acidaemia (pH 7.10) indicate an additional acute respiratory acidosis as a result of the respiratory arrest. In the pre-existing compensated chronic respiratory acidosis, the pH would have been close to normal. Treatment will include, if appropriate, non-invasive ventilation or tracheal intubation and ventilation.
Feedback
That is not right. Have another go.
Feedback
That is not right.
- The patient’s PaO2 is >10 kPa indicating he is not hypoxaemic. However, we would expect a much higher PaO[sub]2[/sub] being ventilated with 85% oxygen. This indicates severely impaired oxygenation
- His pH is 7.10, much lower than normal indicating that he has an acidaemia
- His PaCO[sub]2[/sub] is very high which is consistent with his low pH and so he has a respiratory acidosis. This is due to the period of apnoea and his underlying COPD
- His bicarbonate and base excess are both increased, indicating a metabolic alkalosis. This is a compensatory mechanism for his longstanding COPD. Prior to his respiratory arrest this compensation would have resulted in his pH being almost normal despite an increased PaCO[sub]2[/sub]
In summary:
The significant acidaemia (pH 7.10) indicate an additional acute respiratory acidosis as a result of the respiratory arrest. In the pre-existing compensated chronic respiratory acidosis, the pH would have been close to normal. Treatment will include, if appropriate, non-invasive ventilation or tracheal intubation and ventilation.
Feedback
That is right.
- The patient’s PaO2 is >10 kPa indicating he is not hypoxaemic. However, we would expect a much higher PaO[sub]2[/sub] being ventilated with 85% oxygen. This indicates severely impaired oxygenation
- His pH is 7.10, much lower than normal indicating that he has an acidaemia
- His PaCO[sub]2[/sub] is very high which is consistent with his low pH and so he has a respiratory acidosis. This is due to the period of apnoea and his underlying COPD
- His bicarbonate and base excess are both increased, indicating a metabolic alkalosis. This is a compensatory mechanism for his longstanding COPD. Prior to his respiratory arrest this compensation would have resulted in his pH being almost normal despite an increased PaCO[sub]2[/sub]
In summary:
The significant acidaemia (pH 7.10) indicate an additional acute respiratory acidosis as a result of the respiratory arrest. In the pre-existing compensated chronic respiratory acidosis, the pH would have been close to normal. Treatment will include, if appropriate, non-invasive ventilation or tracheal intubation and ventilation.
References
Essentials: 5-step approach to ABG interpretation
Step 2 – is the patient hypoxaemic?
Step 3 – is the patient acidaemic or alkalaemic?
Step 4 – what happened to the PaCO2?
Step 5 – what has happened to the base excess or bicarbonate?
Normal values
PaO[sub]2[/sub] > 10 kPa (75 mmHg) on air
pH 7.35 – 7.45
PaCO[sub]2[/sub] 4.7 – 6.0 kPa
HCO[sub]3[/sub] 22 – 26 mmol l-1
BE +/- 2 mmol l[sup]-1[/sup]