Ensure post resuscitation care
Post Resuscitation Care
Learning outcomes
To understand:
- The need for continued resuscitation after return of spontaneous circulation
- How to transfer the patient safely
- How to treat the post cardiac arrest syndrome
- The role and limitations of assessing prognosis after cardiac arrest.

Chain of survival
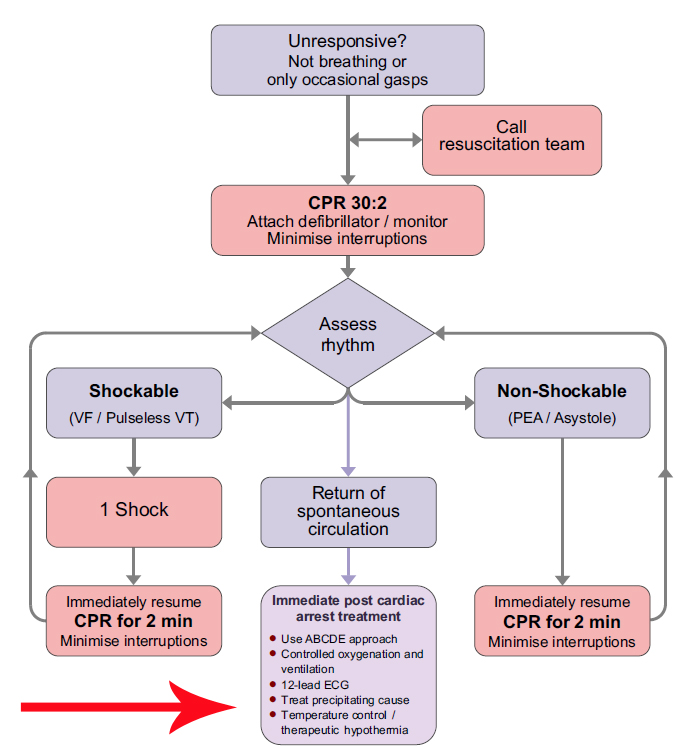
Post resuscitation care
The goal is to restore:
- Normal cerebral function
- Stable cardiac rhythm
- Adequate organ perfusion
- Quality of life
Post cardiac arrest syndrome
- Post cardiac arrest brain injury:
- coma
- seizures
- myoclonus
- Post cardiac arrest myocardial dysfunction
- Systemic ischaemia-reperfusion response
- ‘sepsis-like’ syndrome
- Persistence of precipitating pathology
Airway and breathing
- Ensure a clear airway, adequate oxygenation and ventilation
- Consider tracheal intubation, sedation and controlled ventilation
- Pulse oximetry:
- aim for SpO2 94 – 98%
- Capnography:
- AIM for normocarbia
- AVOID hyperventilation
Airway and breathing
- Look, listen and feel
- Consider:
- simple/tension pneumothorax
- collapse/consolidation
- bronchial intubation
- pulmonary oedema
- aspiration
- fractured ribs/flail segment
Airway and breathing
- Insert gastric tube to decompress stomach and
improve lung compliance - Secure airway for transfer
- Consider immediate extubation if patient breathing and
conscious level improves quickly after ROSC
Circulation
- Pulse and blood pressure
- Peripheral perfusion, e.g. capillary refill time
- Right ventricular failure
- distended neck veins
- Left ventricular failure
- pulmonary oedema
- ECG monitor and 12-lead ECG
Disability
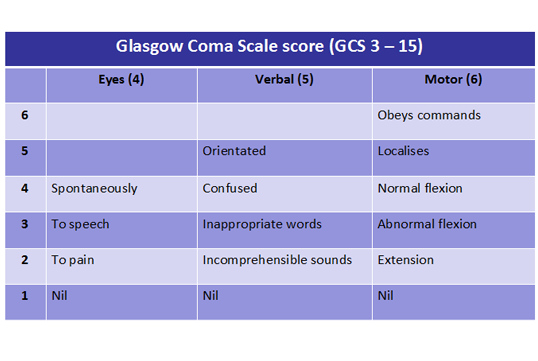
Neurological assessment:
- Glasgow Coma Scale score
- Pupils
- Limb tone and movement
- Posture
Further assessment – History
- Health before the cardiac arrest
- Time delay before resuscitation
- Duration of resuscitation
- Cause of the cardiac arrest
- Family history
Further assessment – Monitoring
- Vital signs
- ECG
- Pulse oximetry
- Blood pressure, e.g. arterial line
- Capnography
- Urine output
- Temperature
Further assessment – Investigations
- Arterial blood gases
- Full blood count
- Biochemistry including blood glucose
- Troponin
- Repeat 12-lead ECG
- Chest X-ray
- Echocardiography
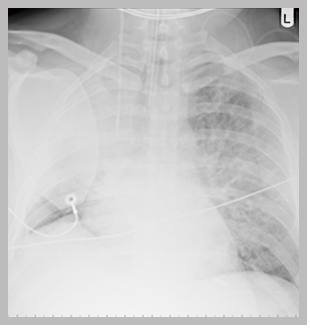
A chest X-ray showing:
- A correctly positioned tracheal tube
- A right internal jugular central line
- A defibrillation patch
- A collapse of the right upper lobe with loss of lung volume on the right
Transfer of the patient
- Discuss with admitting team
- Cannulae, drains, tubes secured
- Suction
- Oxygen supply
- Monitoring
- Documentation
- Reassess before leaving
- Talk to family
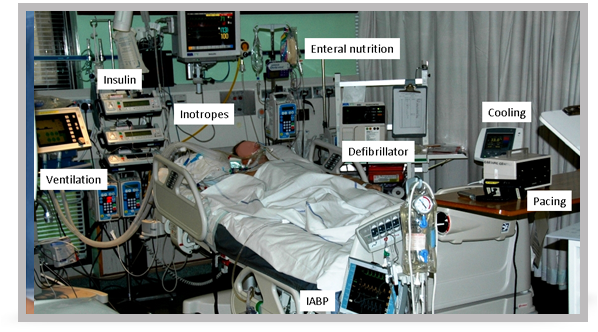
Optimising organ function
Heart
- Post cardiac arrest syndrome
- Ischaemia-reperfusion injury:
- reversible myocardial dysfunction for 2-3 days
- arrhythmias
Optimising organ function
Heart
- Poor myocardial function despite optimal filling:
- echocardiography
- cardiac output monitoring
- inotropes and/or balloon pump
- Mean blood pressure to achieve:
- urine output of 1 ml kg-1 h-1
- normalising lactate concentration
Optimising organ function
Brain
- Impaired cerebral autoregulation – maintain ‘normal’ blood pressure
- Sedation
- Control seizures
- Glucose (4-10 mmol l-1)
- Normocarbia
- Avoid/treat hyperthermia
- Consider therapeutic hypothermia
Therapeutic hypothermia
Who to cool?
- Unconscious adults with ROSC after VF arrest should be cooled to 32-34oC
- May benefit patients after non-shockable/in-hospital cardiac arrest
- Exclusions: severe sepsis, pre-existing medical coagulopathy
- Start as soon as possible and continue for 24 h
- Rewarm slowly 0.25oC h-1
Therapeutic hypothermia
How to cool?
- Induction - 30 ml kg-1 4oC IV fluid and/or external cooling
- Maintenance - external cooling:
- ice packs, wet towels
- cooling blankets or pads
- water circulating gel-coated pads
- Maintenance - internal cooling:
- intravascular heat exchanger
- cardiopulmonary bypass
Therapeutic hypothermia
Physiological effects and complications
- Shivering: sedate +/- neuromuscular blocking drug
- Bradycardia and cardiovascular instability
- Infection
- Hyperglycaemia
- Electrolyte abnormalities
- Impaired coagulation
- Increased amylase values
- Reduced clearance of drugs
Assessment of prognosis
- No clinical neurological signs can predict outcome < 24 h after ROSC
- Poor outcome predicted at 3 days by:
- absent pupil light and corneal reflexes
- absent or extensor motor response to pain
- But limited data on reliability of these criteria after therapeutic hypothermia
Organ donation
- Non-surviving post cardiac arrest patient may be a suitable donor:
- heart-beating donor (brainstem death)
- non-heart-beating donor
Summary
- Post cardiac arrest syndrome is complex
- Quality of post resuscitation care influences final outcome
- Appropriate monitoring, safe transfer and continued organ support
- Assessment of prognosis is difficult
Advanced Life Support Course
E-lecture
All rights reserved
© Resuscitation Council (UK) 2011
1. Welcome to this lecture on post resuscitation care.
2. The learning outcomes for this lecture are to understand:
- The importance of continued resuscitation after return of spontaneous circulation
- How to transfer the patient safely
- How to treat the post cardiac arrest syndrome
- The role and limitations of assessing prognosis after cardiac arrest.
3. Post resuscitation care is the final link in the chain of survival.
The treatment the patient receives after spontaneous circulation has been achieved influences significantly the chances of achieving a good neurological outcome.
4. The goal of post resuscitation care is to restore:
- normal cerebral function
- a stable cardiac rhythm
- perfusion that is enough to maintain organ function
- good quality of life.
5. The post-cardiac-arrest syndrome often complicates the post-resuscitation phase.
This comprises:
- post cardiac arrest brain injury,
- post cardiac arrest myocardial dysfunction,
- the systemic ischaemia/reperfusion response
- persistence of precipitating pathology.
The severity of this syndrome will vary with the duration and cause of cardiac arrest - it may not occur at all if the cardiac arrest is brief.
Post cardiac arrest brain injury manifests as coma, seizures, myoclonus, varying degrees of neurological dysfunction and brain death.
Significant myocardial dysfunction is common after cardiac arrest but typically recovers after 2-3 days.
The whole body ischaemia/reperfusion that occurs after resuscitation from cardiac arrest activates immunological and coagulation pathways that cause multiple organ failure and increase the risk of infection.
The post cardiac arrest syndrome has many features in common with sepsis, including intravascular volume depletion and vasodilation.
Any persisting pathology relating to the cause of the cardiac arrest will also need treatment.
6. Patients who have had a brief period of cardiac arrest and have responded immediately to treatment may achieve an immediate return of normal cerebral function.
These patients do not require tracheal intubation and ventilation, but should be given oxygen by facemask to maintain normal arterial oxygen saturation.
Consider tracheal intubation, sedation, and controlled ventilation in any patient with obtunded cerebral function.
Hypoxaemia and hypercarbia both increase the likelihood of a further cardiac arrest and may contribute to secondary brain injury.
Several animal studies and some limited human data indicate that hyperoxaemia causes oxidative stress and may harm post-ischaemic neurones.
As soon as arterial blood oxygen saturation can be monitored reliably (by blood gas analysis and/or pulse oximetry), adjust the inspired oxygen concentration to maintain the arterial blood oxygen saturation in the range of 94-98%.
Adjust ventilation to achieve normocarbia and monitor this using the end-tidal CO2 with waveform capnography and arterial blood gas values.
7. Examine the patient’s chest and look for symmetrical chest movement.
Listen to ensure that the breath sounds are equal on both sides.
A tracheal tube that has been inserted too far will tend to go down the right main bronchus and fail to ventilate the left lung.
If ribs have been fractured during chest compression there may be a pneumothorax or a flail segment.
Listen for evidence of pulmonary oedema or pulmonary aspiration of gastric contents.
8. Insert a gastric tube - this will decompress the stomach following mouth-to-mouth or bag-mask ventilation, prevent splinting of the diaphragm, and enable drainage of gastric contents.
If the intubated patient regains consciousness soon after ROSC, and is breathing normally, consider immediate extubation. Ensure that a rigid sucker is available.
If immediate or early extubation is not possible, sedate the patient to ensure the tracheal tube is tolerated, and provide ventilatory support.
Ensure the airway is secure before transfer.
9. Cardiac rhythm and haemodynamic function are likely to be unstable following a cardiac arrest.
Look for evidence of poor cardiac function:
- Record the pulse and blood pressure and assess peripheral perfusion; warm, pink digits with a rapid capillary refill usually imply adequate perfusion.
- Grossly distended neck veins when the patient is semi-upright may indicate right ventricular failure, but in rare cases could indicate pericardial tamponade.
- Left ventricular failure may be indicated by fine inspiratory crackles and the production of pink frothy sputum. Once in a high-care area, consider the use of a non-invasive cardiac output monitor.
- Record a 12-lead ECG as soon as possible.
- Acute ST- segment elevation or new left bundle branch block in a patient with a typical history of acute MI is an indication for reperfusion therapy, either with a thrombolytic or by emergency percutaneous coronary intervention.
10. Assess neurological function rapidly by recording:
- the Glasgow Coma Scale score
- the pupillary response to light
- limb tone and posture.
11. The Glasgow Coma Scale comprises three components: eyes, verbal and motor responses.
The maximum score possible is 15; the minimum score possible is 3.
12. Obtain a comprehensive history as soon as possible.
The information may come from relatives, EMS personnel or the GP.
The patient’s baseline physiological reserve (before the cardiac arrest) is one of the most important factors taken into consideration by the ICU team when determining whether prolonged multiple organ support is appropriate.
Determine the delay before the start of resuscitation and the duration of the resuscitation; this may have prognostic significance, although is generally unreliable, and certainly should not be used alone to predict outcome
Consider other causes of cardiac arrest if there is little to suggest primary cardiac disease.
13. Continuous monitoring is essential to detect changes during the period of instability that follows resuscitation from cardiac arrest.
These monitors should include:
- vital signs
- ECG
- pulse oximetry
- blood pressure – continuous monitoring using an arterial line is preferable
- capnography
- urine output
- temperature.
14. Several investigations are indicated in the post resuscitation phase:
- Arterial blood gas analysis is valuable for documenting the severity of the likely metabolic, and probably respiratory, acidosis. The effectiveness of continued resuscitation can be confirmed by documenting reducing lactate values and correction of base deficit.
- A full blood count will exclude anaemia as contributor to myocardial ischaemia and provide baseline values.Check the plasma biochemistry, including blood glucose, and request a troponin.
- Repeat the 12-lead ECG and request a chest X-ray.
- Echocardiography is very useful because it may identify potential causes of cardiac arrest and will enable assessment of ventricular structure and function.
15. This is a chest X-ray taken on the ICU soon after admission, following resuscitation from cardiac arrest.
It shows:
- a correctly positioned tracheal tube
- a right internal jugular central line
- a defibrillation patch
- collapse of the right upper lobe with loss of lung volume on the right.
16. Following the period of initial post resuscitation care and stabilisation, the patient will need to be transferred to an ICU or a CCU.
The decision to transfer the patient should be made only after discussion with senior members of the admitting team.
Continue monitoring during the transfer and secure all cannulae, catheters, tubes and drains.
Ensure that portable suction apparatus, an oxygen supply and a defibrillator/monitor go with the patient.
Make a full re-assessment immediately before the patient is transferred.
Finally, complete all relevant documentation and update the patient’s family as soon as possible.
17. After resuscitation from prolonged cardiac arrest the patient is likely to require multiple organ support such as that pictured in this slide.
18. Post-cardiac arrest myocardial dysfunction is a component of the post cardiac arrest syndrome and causes haemodynamic instability, which manifests as hypotension, a low cardiac output and arrhythmias.
19. Early echocardiography will enable the degree of myocardial dysfunction to be quantified.
In the ICU an arterial line for continuous blood pressure monitoring is essential.
Non-invasive cardiac output monitors may help to guide treatment.
If treatment with fluid resuscitation and vasoactive drugs is insufficient to support the circulation, consider insertion of an intra-aortic balloon pump.
Aim for a mean arterial blood pressure that achieves a urine output of 1 ml per kg per hour and normal or decreasing plasma lactate values, taking into consideration the patient’s normal blood pressure, the cause of the arrest and the severity of any myocardial dysfunction.
20. There are several strategies for optimising neurological recovery:
- Autoregulation of cerebral blood flow is impaired for some time after cardiac arrest, which means that cerebral perfusion varies with blood pressure instead of being linked to neuronal activity.
- Following return of spontaneous circulation, maintain mean arterial pressure near the patient’s normal level.
- The patient is usually sedated with a combination of opioids and hypnotics - short-acting drugs will enable earlier neurological assessment.
- Adequate sedation will reduce oxygen consumption.
- During hypothermia, optimal sedation can reduce or prevent shivering, which enables the target temperature to be achieved more rapidly.
- Seizures occur in 10% to 40% of those who remain comatose.
- Seizures increase cerebral metabolism by up to three times and may cause cerebral injury – they must be treated promptly and effectively with benzodiazepines, phenytoin, sodium valproate, propofol, or a barbiturate.
- Clonazepam is the most effective drug for treating myoclonus.
- There is a strong association between high blood glucose after resuscitation from cardiac arrest and poor neurological outcome. Maintain glucose in the range of 4 – 10 mmol l-1, and be very careful to avoid HYPOglycaemia.
- Maintain normocarbia.
- Hyperthermia is associated with a worse neurological outcome and it should be avoided.
- A period of mild hypothermia may improve neurological outcome.
21. Evidence from two randomised trials supports the use of induced mild hypothermia in comatose survivors of out-of-hospital cardiac arrest caused by VF.
This therapy may benefit patients after non-shockable rhythms or in-hospital cardiac arrest, but the evidence in these patients is more limited.
Cooling should be started as soon as possible after return of spontaneous circulation; the target temperature range of 32-34ºC is maintained for 24 hours before controlled slow rewarm at 0.25ºC per hour.
Recognised exclusion criteria include severe sepsis and pre-existing medical coagulopathy.
22. The practical application of therapeutic hypothermia is divided into three phases: induction, maintenance, and rewarming.
External and/or internal cooling techniques can be used to initiate cooling.
An infusion of 30 ml per kg of ice cold 0.9% sodium chloride or Hartmann’s solution decreases core temperature by approximately 1.5ºC.
Other methods of inducing and/or maintaining hypothermia include:
- (a) external techniques such as:
- simple ice packs and/or wet towels
- cooling blankets or pads
- water or air circulating blankets
- water circulating gel-coated pads.
- (b) internal techniques, such as:
- intravascular heat exchanger, placed usually in a femoral vein
- cardiopulmonary bypass.
23. The well-recognised physiological effects of hypothermia need to be managed carefully.
- Shivering will increase metabolic and heat production, which will reduce cooling rates. Adequate sedation and possibly neuromuscular blocking drugs will be required.
- Mild hypothermia increases systemic vascular resistance and causes arrhythmias - usually bradycardia.
- Hypothermia impairs the immune system increases infection rates.
- Hypothermia decreases insulin sensitivity and insulin secretion, causing hyperglycaemia, which will need treatment with insulin.
- Hypothermia causes a diuresis and electrolyte abnormalities such as hypophosphataemia, hypokalaemia, hypomagnesaemia and hypocalcaemia.
- Mild hypothermia impairs coagulation and increases bleeding.
- The serum amylase concentration is commonly increased during hypothermia but the significance of this is unclear.
- At a core temperature of 34ºC, the clearance of sedative drugs and neuromuscular blockers is reduced by up to 30%.
24. There are no clinical neurological signs that predict reliably poor outcome less than 24 hours after cardiac arrest.
In adult patients who are comatose after cardiac arrest, and who have not been treated with hypothermia, and who do not have confounding factors (such as hypotension, sedatives or muscle relaxants), the absence of both pupillary light and corneal reflex at ≥ 72 hours predicts poor outcome reliably.
A GCS motor score of 2 or less - which is extension or no response to pain - at ≥ 72 hours is less reliable.
Most prognostication studies have been undertaken before implementation of therapeutic hypothermia and this therapy makes these tests less reliable even when undertaken after normothermia has been restored.
Given the limited available evidence, decisions to limit care should not be made based on the results of a single prognostication tool.
25. Post cardiac arrest patients who do not survive represent an opportunity to increase the organ donor pool, either after brain death or as non-heart-beating donors.
- the post cardiac arrest syndrome is complex
- the quality of post-resuscitation care will influence significantly the patient’s final outcome
- these patients require appropriate monitoring, safe transfer to a critical care environment, and continued organ support
- our ability to predict the final neurological outcome for those patients remaining comatose after CPR remains very poor, particularly when mild hypothermia has been used.
Dr Jerry Nolan is a member of the Resuscitation Council (UK) ALS Subcommittee and the immediate past Chair of the Council. He is the Co-Chair of the International Liaison Committee on Resuscitation (ILCOR) and Editor-in-Chief of the journal ‘Resuscitation’. He is a Consultant in Anaesthesia and Intensive Care Medicine at the Royal United Hospital Bath NHS Trust. (2011)